Clsi Mic Breakpoints 2025. Section 3044 of the cures act created a system to expedite the recognition of antimicrobial susceptibility test interpretive criteria also known as “breakpoints” (abbreviated as. The distribution of hundreds of.
Join clsi on april 17 at 1:00 et for a webinar on the latest in antimicrobial susceptibility testing (ast) with the release of the 34th edition of the clsi m100 document. Routine and extended internal quality control for mic determination and disk diffusion as recommended by eucast.
Clsi Breakpoints 2025 E Coli Suzy Leland, Routine and extended internal quality control for mic determination and disk diffusion as recommended by eucast.
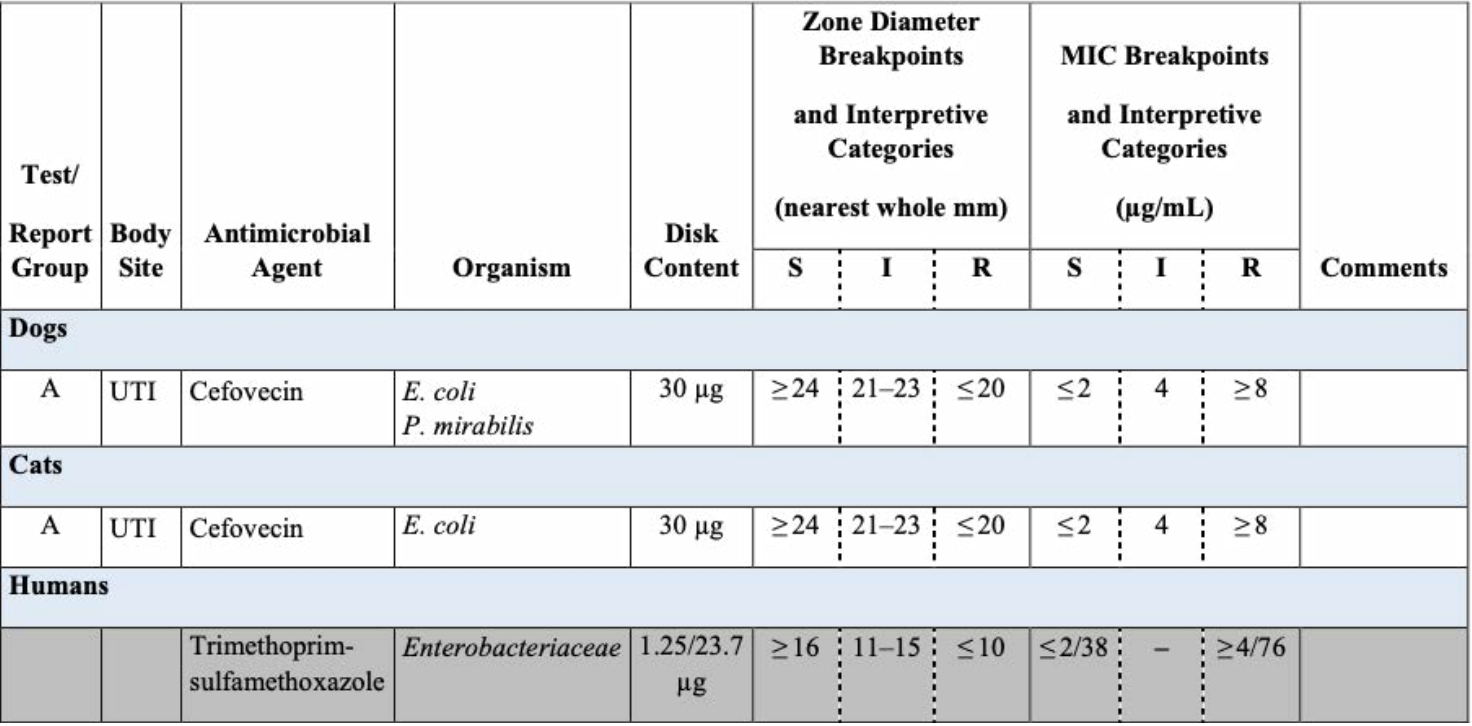
CLSi and eUCASt MiC breakpoints of antimicrobial agents assayed, 1, 2025, laboratories must use current breakpoints to interpret minimum inhibitory concentration (mic) and disk diffusion.

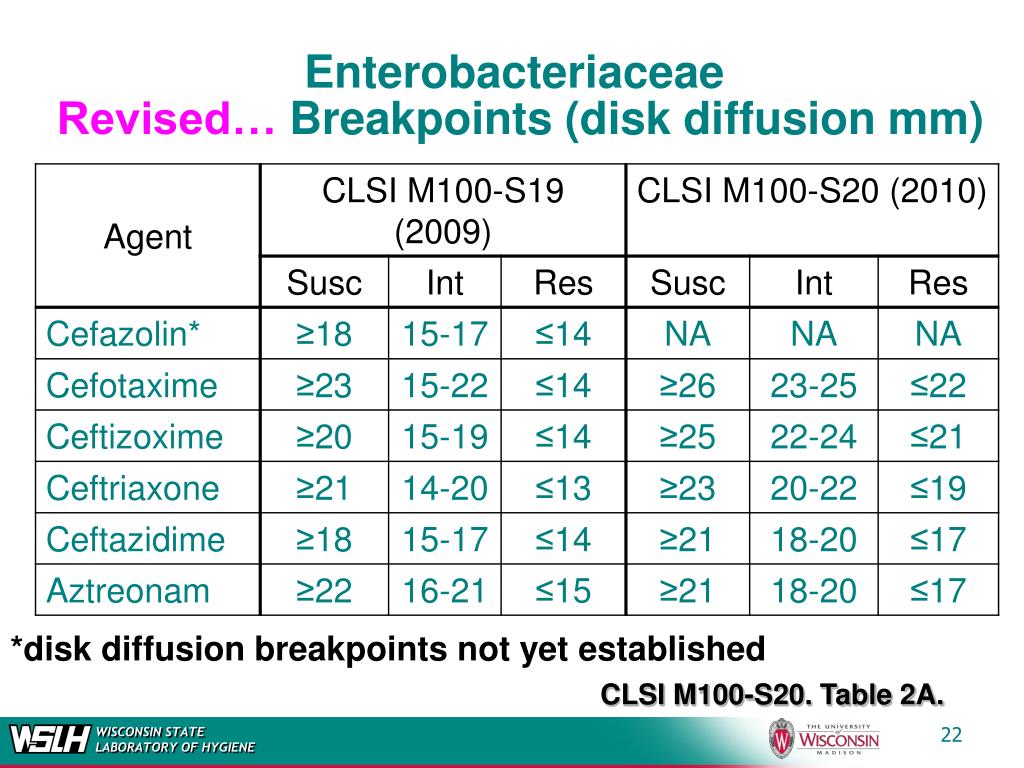
Clsi Breakpoints 2025 E Coli Suzy Leland, M68 aims to provide a protocol for updating breakpoints in a clinical laboratory setting.
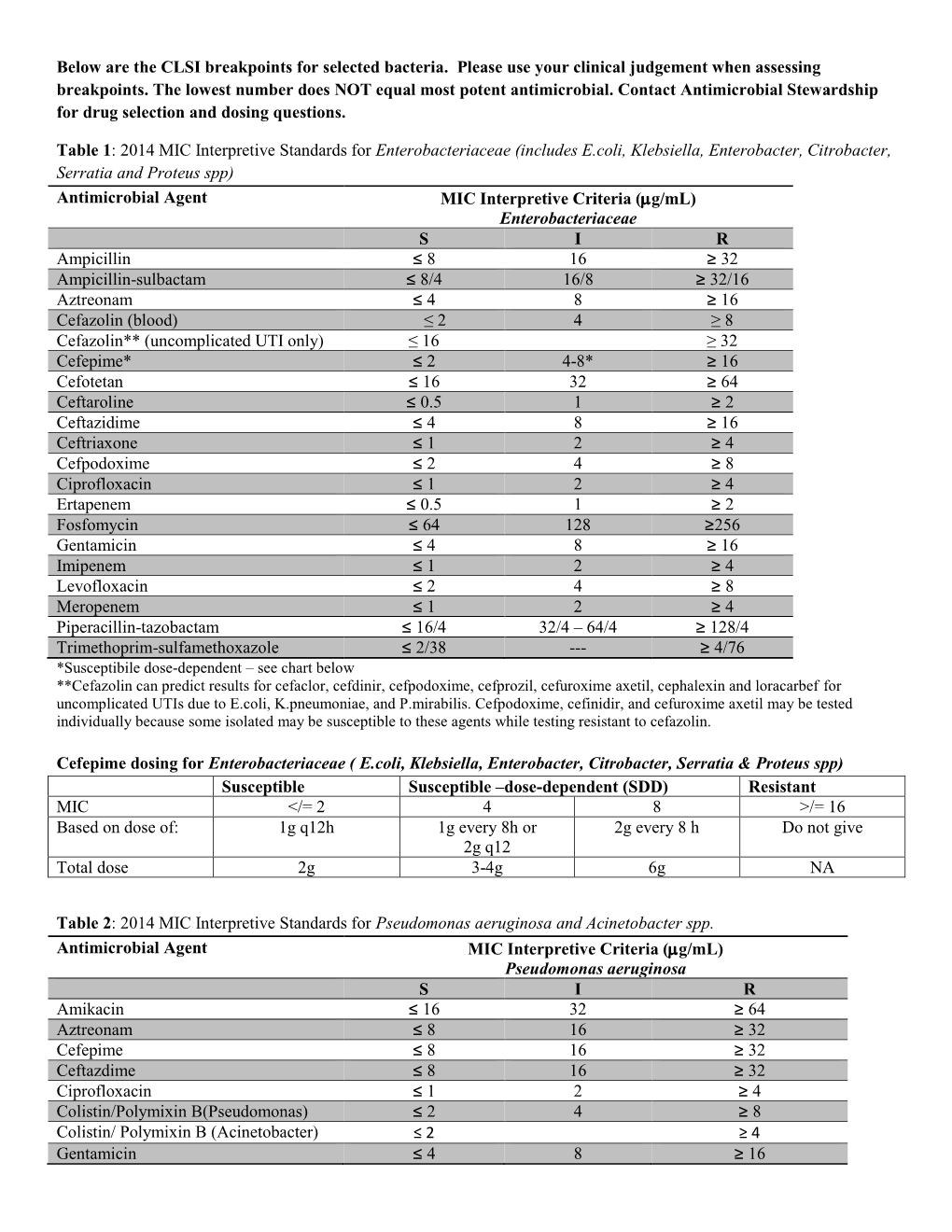
Clsi Breakpoints 2025 E Coli Suzy Leland, M68 aims to provide a protocol for updating breakpoints in a clinical laboratory setting.
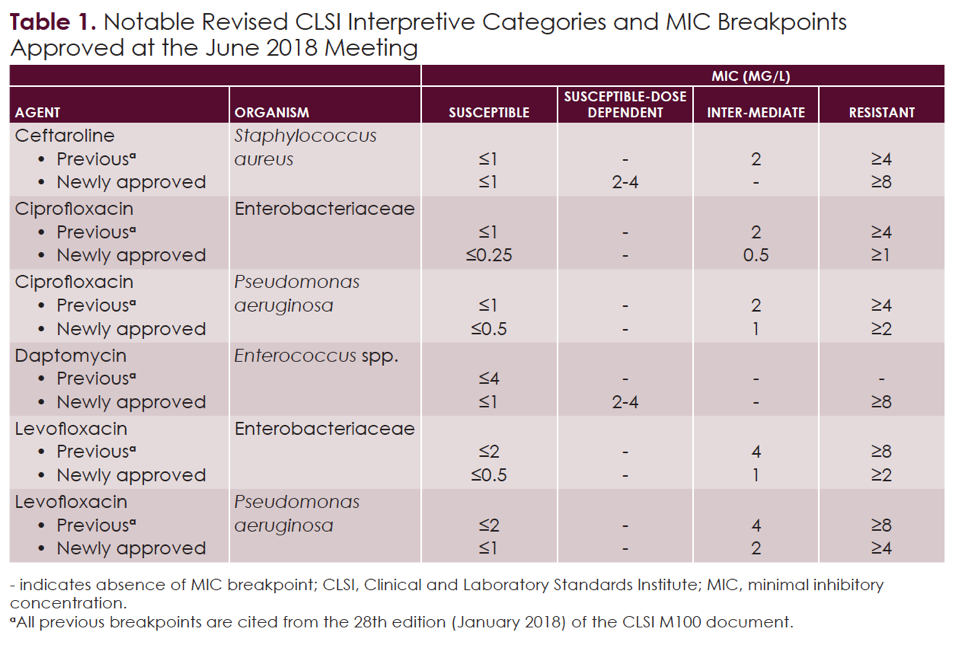
MIC breakpoints for Pseudomonas aeruginosa according to EUCAST and CLSI, Extensive analysis of pk/pd, clinical outcomes, and current susceptibility profiles for enterobacterales and p.

CLSI standard minimal inhibitory concentration (MIC) Download Table, 1, 2025, laboratories must use current breakpoints to interpret minimum inhibitory concentration (mic) and disk diffusion.

MIC breakpoints for S. aureus according to EUCAST and CLSI guidelines, Cap microbiology checklist requirement mic.11385 states that by jan.

List of Antimicrobial Agents and CLSI Breakpoint Interpretative, The breakpoint tables, dosing tab, qc tables and manuals pertaining to disk diffusion updated.

CLSI a minimum inhibitory concen tration (MIC) breakpoints, 1, 2025, laboratories must use current breakpoints to interpret minimum inhibitory concentration (mic) and disk diffusion.

PPT Antibiotics 102 Reading and Interpreting CLSI Antimicrobial, M68 aims to provide a protocol for updating breakpoints in a clinical laboratory setting.